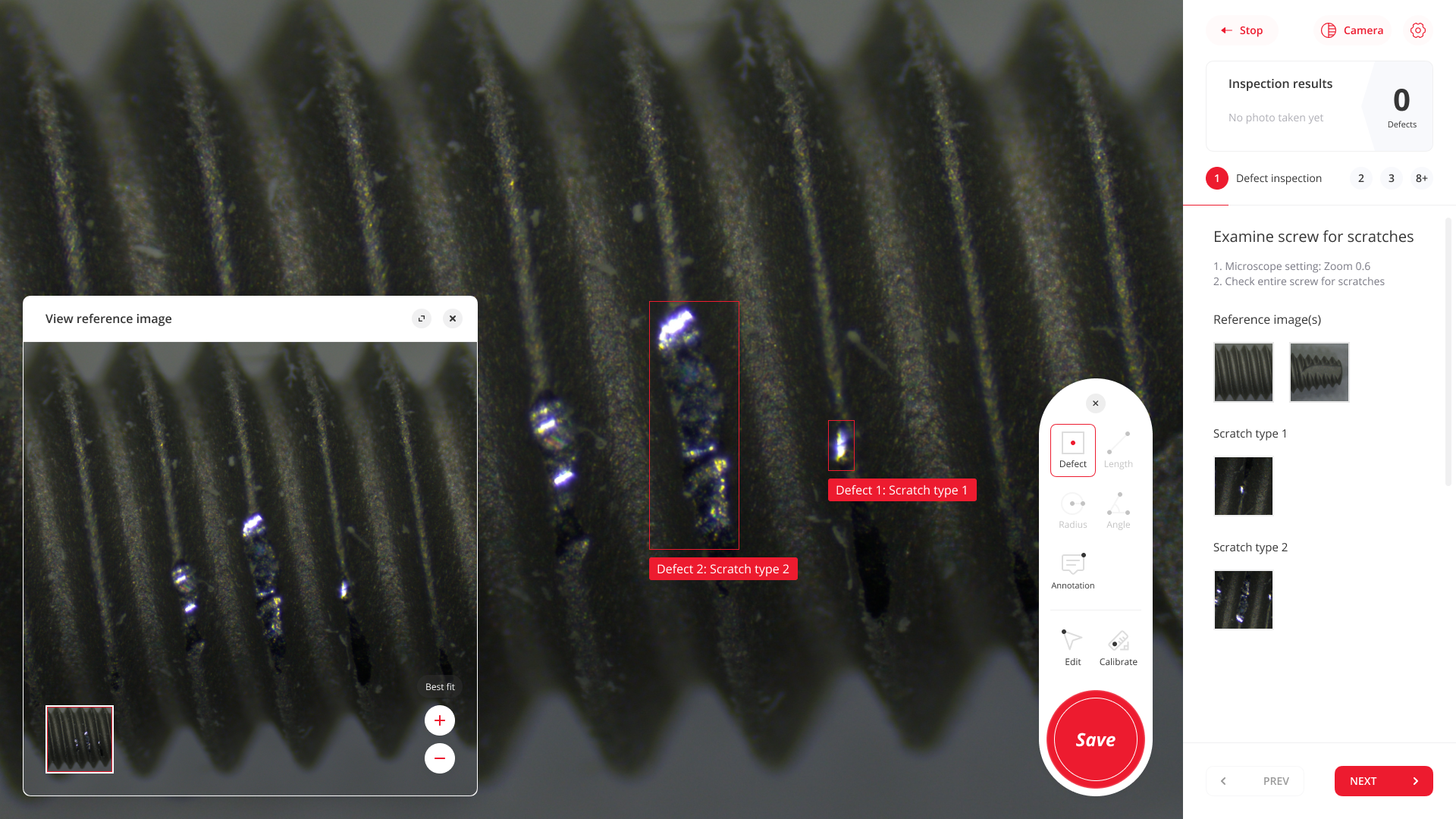
Elevate your inspection workflow for medical devices - digital, consistent and traceable.
Date: 18 May | Time: 11 AM London / 12 PM Berlin / 03 PM Dubai
Elevate your inspection workflow for medical devices - digital, consistent and traceable.
Date: 18 May | Time: 11 AM London / 12 PM Berlin / 03 PM Dubai
Already Registered?
If you have previously registered for this event, please login below:
Register your details below